Evolocumab, a medicine currently used for lowering LDL cholesterol, has a unique protective effect against kidney disease. In laboratory experiments, a novel property of evolocumab was shown to mitigate kidney damage caused by a high-fat diet that can otherwise lead to chronic kidney disease (CKD).
The discovery was made by a team of scientists at the Hamilton Centre for Kidney Research, affiliated with McMaster University and The Research Institute of St. Joe’s Hamilton. Their findings were published on April 27 in the journal Kidney360.
 Jae Hyun Byun and Dr. Richard Austin, part of the study team.
Jae Hyun Byun and Dr. Richard Austin, part of the study team.
Evolocumab is a humanized monoclonal antibody that lowers blood levels of low-density lipoprotein (LDL) cholesterol – considered “bad” cholesterol – by targeting PCSK9. Though it inhibits the interaction between the LDL receptor and PCSK9, evolocumab does not affect PCSK9’s ability to bind to the scavenger receptor CD36, a driver of kidney disease.
PCSK9, a blood protein that circulates throughout the body, is expressed in various organs such as the liver and kidneys. Previous research has shown that PCSK9 interacts with a wide range of receptors known to promote lipid uptake, including both the LDL and CD36 receptors.
In the absence of PCSK9, LDL receptors are free to remove LDL cholesterol from the bloodstream. However, without PCSK9 to inhibit CD36 receptors, higher quantities of fat can accumulate within kidney cells and lead to chronic kidney disease.
“Ordinarily, PCSK9 is a double-edged sword. It can help lower cellular fat accumulation by enhancing the degradation of CD36, yet it reduces the ability to process bad cholesterol because it also binds to LDL receptors,” said Jae Hyun Byun, co-first author of the study. “We have now discovered that evolocumab’s ability to target PCSK9 can reduce both LDL cholesterol and cellular fat intake by decreasing CD36 receptor levels.”
Evolocumab targets PCSK9 by binding to the molecule’s LDL receptor binding region, though its CD36 binding region remains unaffected. This leaves PCSK9 capable of binding to CD36 receptors on kidney cells, reducing CD36-induced intracellular fat accumulation while having no negative effect on LDL cholesterol clearance.
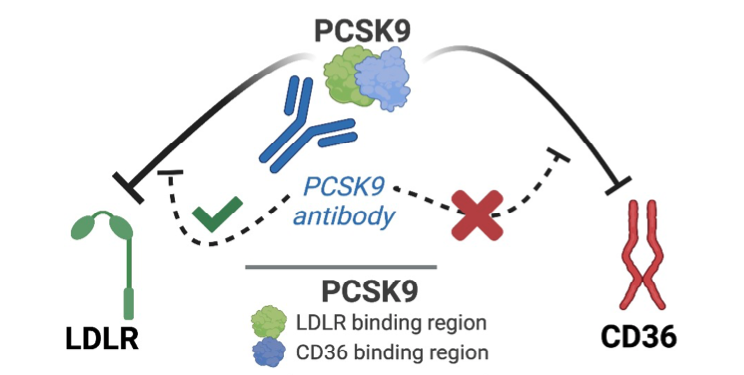 The LDL receptor binding region of PCSK9 is inhibited by the monoclonal antibody evolocumab, while its CD36 binding region remains unaffected.
The LDL receptor binding region of PCSK9 is inhibited by the monoclonal antibody evolocumab, while its CD36 binding region remains unaffected.
A consistent high-fat diet can cause reduced kidney function – a phenomenon known as renal lipotoxicity – that can lead to CKD. As CKD progresses, patients commonly develop interstitial fibrosis (scarring of kidney tissue), proteinuria, and tubular atrophy. Ultimately, these conditions compromise the overall filtration capacity of the kidneys.
In fact, the global prevalence of CKD is increasing, and is associated with a substantial burden on patients and health care systems. Patients with CKD are also at a much higher risk for atherosclerosis and cardiovascular disease.
Within the kidneys at the cellular level, increased uptake of free fatty acids as a result of a high-fat diet causes stress in the endoplasmic reticulum, where proteins are synthesized and folded. Consistent misfolding of proteins within these cells can lead to cell injury and cell death, inflammation, and fibrosis.
“These findings, along with our other investigations into PCSK9, may also lead to the repurposing of this heart medicine for the prevention and treatment of CKD,” said Dr. Richard Austin, senior study author, Director of the Hamilton Centre for Kidney Research, and Professor of Medicine at McMaster University.
The present study and findings represent the latest in a series of discoveries by the Hamilton Centre for Kidney Research related to PCSK9 and other small molecules. For example, the Centre's study on the interaction between caffeine and PCSK9 in the liver was recently published in Nature Communications.
“Our team’s publications on PCSK9 are beginning to come together to tell a beautiful story, as each study provides us with additional information showing the multifaceted roles for PCSK9 and the medicines that target this blood protein,” said Byun.
 Jae Hyun Byun, co-first author of the paper, discussing the findings with Dr. Austin.
Jae Hyun Byun, co-first author of the paper, discussing the findings with Dr. Austin.
Researchers at the Hamilton Centre for Kidney Research continue their novel investigations into PCSK9 and other small molecules as they work to understand the molecular mechanisms involved in chronic kidney disease, atherosclerosis, cardiovascular disease, and many other prevalent conditions.